As a critical fat-soluble secosteroid, Vitamin D3 Powder (Cholecalciferol) serves as more than just a nutrient; it is a precursor to a powerful metabolic hormone. For industry professionals in the food, pharmaceutical, and feed sectors, understanding its chemical behavior and regulatory limits is essential for safe and effective formulation.
1. Chemical Nature and Molecular Origin
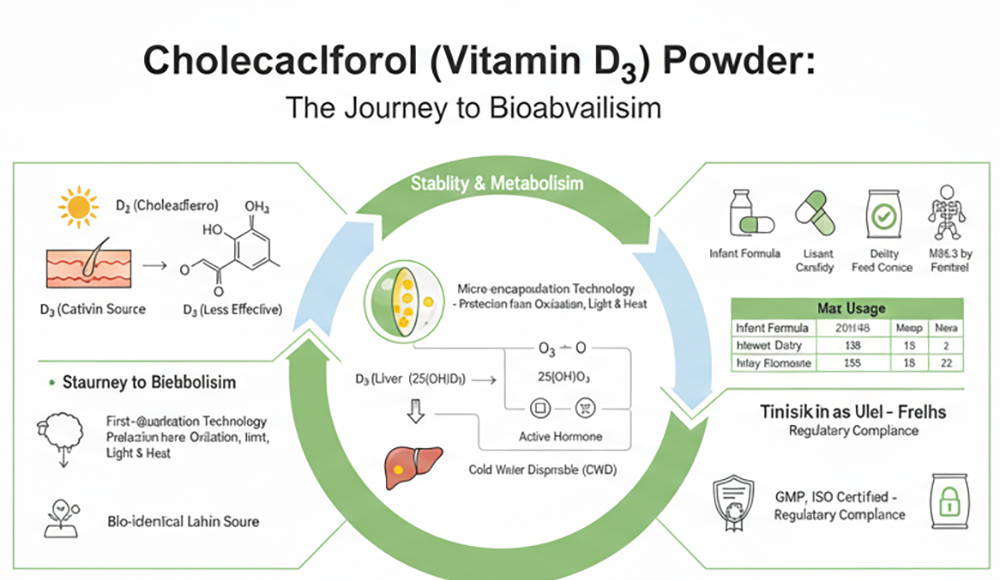
Vitamin D3, with the molecular formula $C_{27}H_{44}O$, is a derivative of cholesterol. In nature, its synthesis occurs via a photochemical reaction. When the skin is exposed to ultraviolet B (UVB) radiation, 7-dehydrocholesterol undergoes a structural transformation into cholecalciferol.
Industrially, Vitamin D3 is primarily synthesized from cholesterol extracted from lanolin. This bio-identical form is preferred over plant-derived Vitamin D2 (ergocalciferol) due to its superior stability and higher efficacy in raising serum 25-hydroxyvitamin D levels in humans.
2. Physiological Role: The Central Regulator of Mineral Homeostasis
The primary function of Vitamin D3 is to maintain the balance of calcium and phosphorus in the blood. Its physiological impact follows three main pathways:
-
Intestinal Absorption: It stimulates the synthesis of calcium-binding proteins in the small intestine, significantly increasing the efficiency of dietary calcium uptake.
-
Bone Mineralization: It works in tandem with Parathyroid Hormone (PTH) to regulate the deposition of calcium salts into the bone matrix, preventing conditions such as rickets in children and osteoporosis in adults.
-
Cellular Modulation: Modern research has identified Vitamin D receptors (VDR) in nearly every tissue, suggesting that Vitamin D3 plays a role in immune function and the regulation of cell proliferation.
3. The Metabolic Journey: A Two-Step Activation
Cholecalciferol itself is biologically inactive. It must undergo two successive hydroxylation steps to become functional:
-
Hepatic Transformation: In the liver, Vitamin D3 is converted into 25-hydroxyvitamin D3 [25(OH)D3] by the enzyme 25-hydroxylase. This is the primary circulating form used by clinicians to measure a patient’s Vitamin D status.
-
Renal Activation: The 25(OH)D3 travels to the kidneys, where it is converted into 1,25-dihydroxyvitamin D3 [1,25(OH)2D3]—the final, hormonally active form.
4. Industrial Applications and Usage
Vitamin D3‘s high biological activity makes it indispensable across multiple sectors:
-
Pharmaceuticals: Used in the treatment of Vitamin D deficiency, hypocalcemia, and metabolic bone diseases.
-
Food Fortification: Added to dairy products, beverages, infant formulas, and margarines.
-
Feed Industry: A core additive in animal nutrition to improve skeletal strength, eggshell quality in poultry, and overall reproductive health.
5. Regulatory Standards: Maximum Usage and Residue Limits
As a fat-soluble nutrient capable of bioaccumulation, the use of Vitamin D3 is strictly governed by international food safety standards (such as GB 2760, USP, and Codex Alimentarius).
| Food Category | Standard Maximum Usage (Approx.) |
| Infant Formula | $0.25\mu g$ – $1.0\mu g$ per 100kJ (strictly regulated) |
| Dairy Products | $10\mu g$ – $40\mu g$ per kg (depending on regional standards) |
| Fortified Beverages | $2\mu g$ – $10\mu g$ per kg |
Regarding Residue Limits: In the context of animal-derived foods (meat, eggs, milk), Vitamin D3 is viewed as a nutritional additive. Therefore, “Maximum Residue Limits” (MRLs) are generally managed through maximum allowable feed inclusion rates. The objective is to ensure the final consumer product does not exceed safe daily intake levels, preventing hypercalcemia.
- Alice Wang
- Whatsapp: +86 133 7928 9277
- Email: info@demeterherb.com
Post time: Jan-16-2026